Methane (CH4)
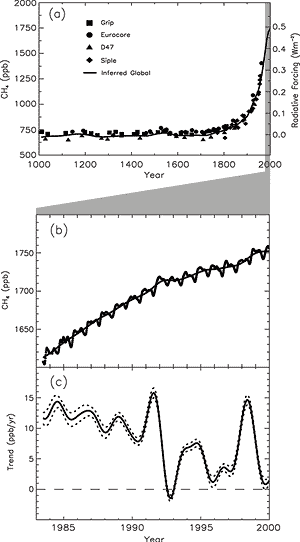
Figure 11: (a) Change in CH4 abundance (mole fraction,
in ppb = 10-9) determined from ice cores, firn, and whole air
samples plotted for the last 1000 years. Radiative forcing, approximated
by a linear scale since the pre-industrial era, is plotted on the right
axis.
(b) Globally averaged CH4 (monthly varying) and deseasonalised
CH4 (smooth line) abundance plotted for 1983 to 1999.
(c) Instantaneous annual growth rate (ppb/yr) in global atmospheric
CH4 abundance from 1983 through 1999 calculated as the derivative
of the deseasonalised trend curve above. Uncertainties (dotted lines) are
±1 standard deviation. [Based on Figure
4.1] |
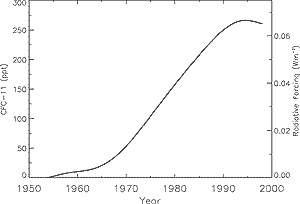
Figure 12: Global mean CFC-11 (CFCl3) tropospheric abundance
(ppt) from 1950 to 1998 based on smoothed measurements and emission models.
CFC-11's radiative forcing is shown on the right axis. [Based on Figure
4.6] |
Atmospheric methane (CH4) concentrations have increased by about
150% (1,060 ppb) since 1750. The present CH4 concentration has
not been exceeded during the past 420,000 years. Methane (CH4) is a
greenhouse gas with both natural (e.g., wetlands) and human-influenced sources
(e.g., agriculture, natural gas activities, and landfills). Slightly more than
half of current CH4 emissions are anthropogenic. It is removed from
the atmosphere by chemical reactions. As Figure 11
shows, systematic, globally representative measurements of the concentration of
CH4 in the atmosphere have been made since 1983, and the record of
atmospheric concentrations has been extended to earlier times from air extracted
from ice cores and firn layers. The current direct radiative forcing of 0.48 Wm-2
from CH4 is 20% of the total from all of the long-lived and globally
mixed greenhouse gases (see Figure 9).
The atmospheric abundance of CH4 continues to increase, from
about 1,610 ppb in 1983 to 1,745 ppb in 1998, but the observed annual increase
has declined during this period. The increase was highly variable during
the 1990s; it was near zero in 1992 and as large as 13 ppb during 1998. There
is no clear quantitative explanation for this variability. Since the SAR, quantification
of certain anthropogenic sources of CH4, such as that from rice production,
has improved.
The rate of increase in atmospheric CH4 is due to a small imbalance
between poorly characterised sources and sinks, which makes the prediction of
future concentrations problematic. Although the major contributors to the
global CH4 budget likely have been identified, most of them are quite
uncertain quantitatively because of the difficulty in assessing emission rates
of highly variable biospheric sources. The limitations of poorly quantified
and characterised CH4 source strengths inhibit the prediction of
future CH4 atmospheric concentrations (and hence its contribution
to radiative forcing) for any given anthropogenic emission scenario, particularly
since both natural emissions and the removal of CH4 can be influenced
substantially by climate change.
Nitrous oxide (N2O)
The atmospheric concentration of nitrous oxide (N2O) has steadily
increased during the Industrial Era and is now 16% (46 ppb) larger than in 1750.
The present N2O concentration has not been exceeded during at least
the past thousand years. Nitrous oxide is another greenhouse gas with both natural
and anthropogenic sources, and it is removed from the atmosphere by chemical reactions.
Atmospheric concentrations of N2O continue to increase at a rate of
0.25%/yr (1980 to 1998). Significant interannual variations in the upward trend
of N2O concentrations are observed, e.g., a 50% reduction in annual
growth rate from 1991 to 1993. Suggested causes are several-fold: a decrease in
use of nitrogen-based fertiliser, lower biogenic emissions, and larger stratospheric
losses due to volcanic-induced circulation changes. Since 1993, the growth of
N2O concentrations has returned to rates closer to those observed during
the 1980s. While this observed multi-year variance has provided some potential
insight into what processes control the behaviour of atmospheric N2O,
the multi-year trends of this greenhouse gas remain largely unexplained.
The global budget of nitrous oxide is in better balance than in the SAR,
but uncertainties in the emissions from individual sources are still quite large.
Natural sources of N2O are estimated to be approximately 10 TgN/yr
(1990), with soils being about 65% of the sources and oceans about 30%. New,
higher estimates of the emissions from anthropogenic sources (agriculture, biomass
burning, industrial activities, and livestock management) of approximately 7
TgN/yr have brought the source/sink estimates closer in balance, compared with
the SAR. However, the predictive understanding associated with this significant,
long-lived greenhouse gas has not improved significantly since the last assessment.
The radiative forcing is estimated at 0.15 Wm-2, which is 6% of the
total from all of the long-lived and globally mixed greenhouse gases (see Figure
9).
Halocarbons and related compounds
The atmospheric concentrations of many of those gases that are both ozone-depleting
and greenhouse gases are either decreasing (CFC-11, CFC-113, CH3CCl3
and CCl4) or increasing more slowly (CFC-12) in response to reduced
emissions under the regulations of the Montreal Protocol and its Amendments.
Many of these halocarbons are also radiatively effective, long-lived greenhouse
gases. Halocarbons are carbon compounds that contain fluorine, chlorine,
bromine or iodine. For most of these compounds, human activities are the sole
source. Halocarbons that contain chlorine (e.g., chlorofluorocarbons - CFCs)
and bromine (e.g., halons) cause depletion of the stratospheric ozone layer
and are controlled under the Montreal Protocol. The combined tropospheric abundance
of ozone-depleting gases peaked in 1994 and is slowly declining. The atmospheric
abundances of some of the major greenhouse halocarbons have peaked, as shown
for CFC-11 in Figure 12. The concentrations of CFCs
and chlorocarbons in the troposphere are consistent with reported emissions.
Halocarbons contribute a radiative forcing of 0.34 Wm-2, which is
14% of the radiative forcing from all of the globally mixed greenhouse gases
(Figure 9).
The observed atmospheric concentrations of the substitutes for the CFCs
are increasing, and some of these compounds are greenhouse gases. The abundances
of the hydrochlorofluorocarbons (HCFCs) and hydrofluorocarbons (HFCs) are increasing
as a result of continuation of earlier uses and of their use as substitutes
for the CFCs. For example, the concentration of HFC-23 has increased by more
than a factor of three between 1978 and 1995. Because current concentrations
are relatively low, the present contribution of HFCs to radiative forcing is
relatively small. The present contribution of HCFCs to radiative forcing is
also relatively small, and future emissions of these gases are limited by the
Montreal Protocol.
The perfluorocarbons (PFCs, e.g., CF4 and C2F6)
and sulphur hexafluoride (SF6) have anthropogenic sources, have extremely
long atmospheric residence times, and are strong absorbers of infrared radiation.
Therefore, these compounds, even with relatively small emissions, have the potential
to influence climate far into the future. Perfluoromethane (CF4)
resides in the atmosphere for at least 50,000 years. It has a natural background;
however, current anthropogenic emissions exceed natural ones by a factor of
1,000 or more and are responsible for the observed increase. Sulphur hexafluoride
(SF6) is 22,200 times more effective a greenhouse gas than CO2
on a per-kg basis. The current atmospheric concentrations are very small (4.2
ppt), but have a significant growth rate (0.24 ppt/yr). There is good agreement
between the observed atmospheric growth rate of SF6 and the emissions
based on revised sales and storage data.
|

