4.2.1.3 Hydrofluorocarbons (HFCs)
The HFCs with the largest measured atmospheric abundances are (in order), HFC-23
(CHF3), HFC-134a (CF3CH2F), and HFC-152a (CH3CHF2).
The recent rises in these HFCs are shown in Figure 4.3
along with some major HCFCs, the latter being controlled under the Montreal
Protocol and its Amendments. HFC-23 is a by-product of HCFC-22 production. It
has a long atmospheric lifetime of 260 years, so that most emissions, which
have occurred over the past two decades, will have accumulated in the atmosphere.
Between 1978 and 1995, HFC-23 increased from about 3 to 10 ppt; and it continues
to rise even more rapidly (Oram et al., 1996). HFC-134a is used primarily as
a refrigerant, especially in car air conditioners. It has an atmospheric lifetime
of 13.8 years, and its annual emissions have grown from near zero in 1990 to
an estimated 0.032 Tg/yr in 1996. The abundance continues to rise almost exponentially
as the use of this HFC increases (Montzka et al., 1996b; Oram et al., 1996;
Simmonds et al., 1998). HFC-152a is a short-lived gas with a mean atmospheric
lifetime of 1.4 years. Its rise has been steady, but its low emissions and a
short lifetime have kept its abundance below 1 ppt.
4.2.1.4 Perfluorocarbons (PFCs) and sulphur hexafluoride
(SF6)
PFCs, in particular CF4 and C2F6, as well
as SF6 have sources predominantly in the Northern Hemisphere, atmospheric
lifetimes longer than 1,000 years, and large absorption cross-sections for terrestrial
infra-red radiation. These compounds are far from a steady state between sources
and sinks, and even small emissions will contribute to radiative forcing over
the next several millennia. Current emissions of C2F6
and SF6 are clearly anthropogenic and well quantified by the accumulating
atmospheric burden. Harnisch and Eisenhauer (1998) have shown that CF4
and SF6 are naturally present in fluorites, and out-gassing from
these materials leads to natural background abundances of 40 ppt for CF4
and 0.01 ppt for SF6. However, at present the anthropogenic emissions
of CF4 exceed the natural ones by a factor of 1,000 or more and are
responsible for the rapid rise in atmospheric abundance. Atmospheric burdens
of CF4 and SF6 are increasing as shown in Figures
4.4 and 4.5, respectively. Surface measurements
show that SF6 has increased by about 7%/yr during the 1980s and 1990s
(Geller et al., 1997; Maiss and Brenninkmeijer, 1998). Recent relative rates
of increase are 1.3%/yr for CF4 and 3.2%/yr for C2F6
(Harnisch et al., 1996). The only important sinks for PFCs and SF6
are photolysis or ion reactions in the mesosphere. These gases provide useful
tracers of atmospheric transport in both troposphere and stratosphere.
A new, long-lived, anthropogenic greenhouse gas has recently been found in
the atmosphere (Sturges et al., 2000). Trifluoromethyl sulphur pentafluoride
(SF5CF3) – a hybrid of PFCs and SF6 not
specifically addressed in Annex A of the Kyoto Protocol – has the largest
radiative forcing, on a per molecule basis, of any gas found in the atmosphere
to date. Its abundance has grown from near zero in the late 1960s to about 0.12
ppt in 1999.
|
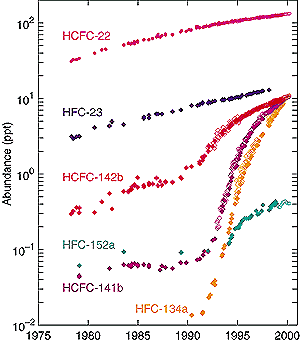
Figure 4.3: HFC-23 (blue, UEA scale), -152a (green, UEA scale),
-134a (orange, NOAA scale), and HCFC-22 (magenta, SIO scale), -142b (red,
NOAA scale), and -141b (purple, NOAA scale) abundances (ppt) at Cape Grim,
Tasmania for the period 1978 to 1999. Different symbols are data from
different measurement networks: SIO (filled circles), NOAA-CMDL (open
diamonds, Montzka et al., 1994, 1996a,b, 1999), UEA (filled diamonds,
Oram et al., 1995, 1996, 1998, 1999) and AGAGE (open circles, only for
1998 to 2000, all gases but HFC-23, Miller et al., 1998; Sturrock et al.,
1999; Prinn et al., 2000). Southern Hemisphere values (Cape Grim) are
slightly lower than global averages.
|
|