4.2.6.2 Atmospheric measurements and modelling of photochemistry
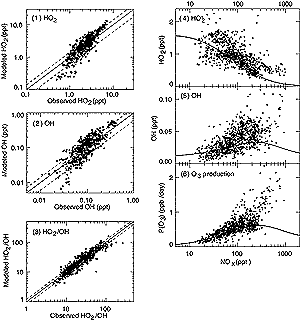
Figure 4.9: (left panel) Observed versus modelled (1) HO2
abundance (ppt), (2) OH abundance (ppt), and (3) HO2/OH ratio
in the upper troposphere (8 to 12 km altitude) during SONEX. Observations
are for cloud-free, daytime conditions. Model calculations are constrained
with local observations of the photochemical background (H2O2,
CH3OOH, NO, O3, H2O, CO, CH4,
ethane, propane, acetone, temperature, pressure, aerosol surface area and
actinic flux). The 1:1 line (solid) and instrumental accuracy range (dashed)
are shown. Adapted from Brune et al. (1999). (right panel) Observed (4)
HO2 abundance (ppt), (5) OH abundance (ppt), and (6) derived
O3 production rate (ppb/day) as a function of the NOx
(NO+NO2) abundance (ppt). Data taken from SONEX (8 to 12 km altitude,
40° to 60°N latitude) and adapted from Jaeglé et al. (1999).
All values are 24-hour averages. The lines correspond to model-calculated
values as a function of NOx using the median photochemical background
during SONEX rather than the instantaneous values (points). |
Atmospheric measurements provide another cornerstone for the
numerical modelling of photochemistry. Over the last five years direct atmospheric
measurements of HOx radicals, made simultaneously with the other key
species that control HOx, have been conducted over a wide range of
conditions: the upper troposphere and lower stratosphere (e.g., SPADE, ASHOE/MAESA,
STRAT; SUCCESS, SONEX, PEM-TROPICS A & B), the remote Pacific (MLOPEX), and
the polluted boundary layer and its outflow (POPCORN, NARE, SOS). These intensive
measurement campaigns provide the first thorough tests of tropospheric OH chemistry
and production of O3 for a range of global conditions. As an example
here, we present an analysis of the 1997 SONEX (Subsonic assessment program Ozone
and Nitrogen oxide EXperiment) aircraft campaign over the North Atlantic that
tests one of the chemical models from the OxComp workshop (HGIS).
The 1997 SONEX aircraft campaign over the North Atlantic provided the first
airborne measurements of HOx abundances concurrent with the controlling
chemical background: H2O2, CH3OOH, CH2O,
O3, NOx, H2O, acetone and hydrocarbons. These
observations allowed a detailed evaluation of our understanding of HOx
chemistry and O3 production in the upper troposphere. Figure
4.9 (panels 1-3) shows a comparison between SONEX measurements and model
calculations (Jaeglé et al., 1999) for OH and HO2 abundances
and the ratio HO2/OH. At each point the model used the local, simultaneously
observed chemical abundances. The cycling between OH and HO2 takes
place on a time-scale of a few seconds and is mainly controlled by reaction
of OH with CO producing HO2, followed by reaction of HO2
with NO producing OH. This cycle also leads to the production of ozone. As seen
in Figure 4.9, the HO2/OH ratio is reproduced
by model calculations to within the combined uncertainties of observations (±20%)
and those from propagation of rate coefficient errors in the model (±100%),
implying that the photochemical processes driving the cycling between OH and
HO2 appear to be understood (Wennberg et al., 1998; Brune et al.,
1999). The absolute abundances of OH and HO2 are matched by model
calculations to within 40% (the reported accuracy of the HOx observations)
and the median model-to-observed ratio for HO2 is 1.12. The model
captures 80% of the observed variance in HOx, which is driven by
the local variations in NOx and the HOx sources (Faloona
et al., 2000, Jaeglé et al., 2000;). The predominant sources of HOx
during SONEX were reaction of O(1D) with H2O and photodissociation
of acetone; the role of H2O2 and CH3OOH as
HOx sources was small. This was not necessarily the case in some
of the other airborne campaigns, where large differences between measured and
modelled OH, up to a factor of 5, were observed in the upper troposphere. In
these campaigns the larger measured OH concentrations were tentatively ascribed
to enhanced levels of OH precursors, such as H2O2, CH3OOH,
or CH2O, whose concentrations had not been measured.
Tropospheric O3 production is tightly linked to the abundance of
NOx, and Figure 4.9 (panel 6) shows this production
rate (calculated as the rate of the reaction of HO2 with NO) for
each set of observations as function of NOx during the SONEX mission.
Also shown in Figure 4.9 (panels 4-5) are the measured
abundances of OH and HO2 as a function of NOx. The smooth
curve on each panel 4-6 is a model simulation of the expected relationship if
the chemical background except for NOx remained unchanged at the
observed median abundances. This curve shows the “expected” behaviour
of tropospheric chemistry when only NOx is increased: OH increases
with NOx abundances up to 300 ppt because HO2 is shifted
into OH; it decreases with increasing NOx at higher NOx
abundances because the OH reaction with NO2 forming HNO3
becomes the dominant sink for HOx radicals. Production of O3
is expected to follow a similar pattern with rates suppressed at NOx
abundances greater than 300 ppt under these atmospheric conditions (e.g., Ehhalt,
1998). These SONEX observations indicate, however, that both OH abundance and
O3 production may continue to increase with NOx concentrations
up to 1,000 ppt because the high NOx abundances were often associated
with convection and lightning events and occurred simultaneously with high HOx
sources. By segregating observations according to HOx source strengths,
Jaeglé et al. (1999) identified the approach to NOx-saturated
conditions predicted by the chemical models when HOx sources remain
constant. A NOx-saturated environment was clearly found for the POPCORN
(Photo-Oxidant formation by Plant emitted Compounds and OH Radicals in north-eastern
Germany) boundary layer measurements in Germany (Rohrer et al., 1998; Ehhalt
and Rohrer, 2000). The impact of NOx-saturated conditions on the
production of O3 is large in the boundary layer, where much of the
NOx is removed within a day, but may be less important in the upper
troposphere, where the local lifetime of NOx is several days and
the elevated abundances of NOx are likely to be transported and diluted
to below saturation levels. This effective reduction of the NOx-saturation
effect due to 3-D atmospheric mixing is seen in the CTM modelling of aviation
NOx emissions where a linear increase in tropospheric O3
is found, even with large NOx emissions in the upper troposphere
(Isaksen and Jackman, 1999).
|