|
3.2.3. Soot and Metal Particles
3.2.3.1. Soot
Aircraft jet engines directly emit solid soot particles. Soot encompasses all
primary, carbon-containing products from incomplete combustion processes in
the engine. Besides the pure (optically black) carbon fraction, these products
may also contain nonvolatile (gray) organic compounds (e.g., Burtscher, 1992;
Bockhorn, 1994). Soot parameters of importance for understanding plume processes
are concentration and size distribution at the engine exit, nucleating and chemical
properties, and freezing ability.
Soot emissions for current aircraft engines are specified under the International
Civil Aviation Organization (ICAO) using smoke number measurements (Chapter
7). The smoke number is dominated by the largest soot particles collected
onto a filter. Sampling soot particles smaller than about 300 nm on such filters
becomes inefficient. Correlations between smoke number and soot mass concentrations
(e.g., Champagne, 1988) are used to estimate the soot mass EI from ICAO certification
data. A mean value has been estimated to be approximately 0.04 g/kg fuel for
the present fleet (Döpelheuer, 1997). Because soot emissions depend strongly
on engine types, power settings, and flight levels, additional information is
generally needed to relate smoke number to emissions under flight conditions.
However, details of the size distribution and physicochemical properties of
soot under flight conditions are generally not known and cannot be inferred
from smoke number data. Soot particle measurements for a variety of contemporary
engines show values that scatter around 1015/kg fuel (Figure
3-3b). Thus, soot is about 100 times less abundant in the plume than volatile
aerosol particles; no significant, if any, dependence exists between soot and
fuel sulfur content (Petzold et al., 1997, 1999; Anderson et al., 1998a; Paladino
et al., 1998). Values of 1014 to 1015/kg fuel are consistent with a mass range
of 0.01 to 0.2 g/kg fuel for individual engines using estimated size distributions.
The older Concorde and T-38 engines show exceptionally high number EIs, whereas
one modern subsonic engine emits much fewer soot particles (about 1013/kg fuel)
(Howard et al., 1996). Soot particles are composed of individual, nearly spherical
particles (spherules), which have a number mean radius between 10 and 30 nm
and exceed the size of volatile aerosol particles in a young plume (Hagen et
al., 1992; Rickey, 1995) (Figure 3-2). Several spherical
soot particles may aggregate and form a complex chain structure that may change
with time (Goldberg, 1985). The smallest soot particles will be most rapidly
immersed in background aerosol droplets by coagulation, consistent with the
fact that only larger individual soot particles or agglomerates with radii larger
than about 50 to 100 nm are observable at cruising levels (Sheridan et al.,
1994). Reported estimates of soot surface area at the engine exit are in the
range of 5,000 to 105 µm2 cm-3 (Rickey, 1995; Petzold et al., 1999). These values
continually decrease as the plume dilutes.
Much less information is available concerning the hydration properties of exhaust
soot. In the initial stages of formation, graphite-like soot particles are hydrophobic.
However, laboratory observations have shown that n-hexane soot particles and
other black carbons are partially hydrated (e.g., Chughtai et al., 1996). Soot
particles fresh from jet engines very likely become hydrophilic by oxidation
processes or deposition of water-soluble species present in the exhaust. Irregular
surface features and chemically active sites can also increase chemical reactivity
and amplify heterogeneous nucleation processes.
A clear correlation between fuel sulfur content and soluble mass fractions
found on fresh exhaust soot suggests that soot hydrates more effectively with
increasing EI(S) and that H2SO4 is the primary soluble constituent (Whitefield
et al., 1993). Hydration of carbon particles was observed under water-subsaturated
conditions after treatment with gaseous H2SO4 (Wyslouzil et al., 1994). This
increase in H2O adsorption is in qualitative agreement with an analysis of the
wetting behavior of graphitic carbon under plume conditions (Kärcher et al.,
1996b). Heterogeneous nucleation of H2SO4 hydrates on soot was found to be unlikely
under plume conditions. Soot hydration properties may also change after treatment
with OH and ozone (Kärcher et al., 1996b; Kotzick et al., 1997).
Production of water-soluble material by the interaction of soot with SO2 is
unlikely because the sticking probabilities of gaseous SO2 on amorphous carbon
are too small (Andronache and Chameides, 1997; Rogaski et al., 1997). However,
SO3 and H2SO4 might easily adsorb on soot prior to volatile particle formation
and may explain measured soluble mass fractions on soot (Kärcher, 1998b). Sulfur
may also become incorporated into soot already within the engines, possibly
via S-containing hydrocarbons involved in soot formation (Petzold and Schröder,
1998). Scavenging of small volatile droplets constitutes another soot activation
pathway (Zhao and Turco, 1995; Brown et al., 1996b; Schumann et al., 1996).
The resulting liquid H2SO4/H2O coating increases with plume age and may enhance
the ice-forming ability of soot, which is only poorly known (Section
3.2.4), or it may suppress reactions identified in the laboratory using
dry soot surfaces (Gao et al., 1998).
3.2.3.2 Metal Particles
Aircraft jet engines also directly emit metal particles. Their sources include
engine erosion and the combustion of fuel containing trace metal impurities
or metal particles that enter the exhaust with the fuel (Chapter
7). Metal particles-comprising elements such as Al, Ti, Cr, Fe, Ni, and
Ba-are estimated to be present at the parts per billion by volume (ppbv) level
at nozzle exit planes (CIAP, 1975; Fordyce and Sheibley, 1975). The corresponding
concentrations of 107 to 108 particles/kg fuel (assuming 1-mm radius; see below)
are much smaller than for soot. Although metals have been found as residuals
in cirrus and contrail ice particles (Chen et al., 1998; Petzold et al., 1998;
Twohy and Gandrud, 1998), their number and associated mass are considered too
small to affect the formation or properties of more abundant volatile and soot
plume aerosol particles.
3.2.4. Contrail and Ice Particle Formation
3.2.4.1. Formation Conditions and Observations
Contrails consist of ice particles that mainly nucleate on exhaust soot and
volatile plume aerosol particles. Contrail formation is caused by the increase
in relative humidity (RH) that occurs in the engine plume as a result of mixing
of warm and moist exhaust gases with colder and less humid ambient air (Schmidt,
1941; Appleman, 1953). The RH with respect to liquid water must reach 100% in
the young plume behind the aircraft for contrail formation to occur (Höhndorf,
1941; Appleman, 1953; Busen and Schumann, 1995; Jensen et al., 1998a). The thermodynamic
relation for formation depends on pressure, temperature, and RH at a given flight
level; fuel combustion properties in terms of the emission index of H2O
and combustion heat; and overall efficiency h (Cumpsty, 1997). h, defined as
the fraction of fuel combustion heat that is used to propel the aircraft, can
be computed from engine and aircraft properties (Schumann, 1996a; see also Section
3.7). Only the fraction (1-h) of the combustion heat leaves the engine with
the exhaust gases. As the value of h increases, exhaust plume temperatures decrease
for a given concentration of emitted water vapor, hence contrails form at higher
ambient temperatures and over a larger range of altitudes in the atmosphere
(Schmidt, 1941). Several recent studies reported formation and visibility of
contrails at temperatures and humidities as predicted by thermodynamic theory
for a variety of aircraft and ambient conditions (Busen and Schumann, 1995;
Schumann, 1996b; Schumann et al., 1996; Jensen et al., 1998a; Petzold et al.,
1998). These data are compiled in Figure 3-4. The
mixing process in the expanding exhaust plume is close to isobaric, so the specific
excess enthalpy and water content of the plume decrease with a fixed ratio as
plume species dilute from engine exit to ambient values. Hence, plume conditions
follow straight "mixing lines" in a plot of H2O
partial pressures versus temperature (Schmidt, 1941) (Figure
3-4). The thermodynamic properties of H2O
are such that the saturation pressures over liquid water and water-ice (solid
and dashed lines) increase exponentially with temperature. Therefore, within
the first second in the plume, the exhaust RH increases to a maximum, then decreases
to ambient values. The ambient temperature reaches threshold values for contrail
formation when the mixing lines touch the liquid saturation curve in Figure
3-4b. Contrails persist when mixing-line endpoints fall between the liquid
and ice saturation pressures-that is, when the ambient atmosphere is ice-supersaturated.
Without ambient ice supersaturation, contrail ice crystals evaporate on time
scales of seconds to minutes. Short-lived contrails may also form without ambient
water vapor if ambient temperatures are sufficiently low.
|
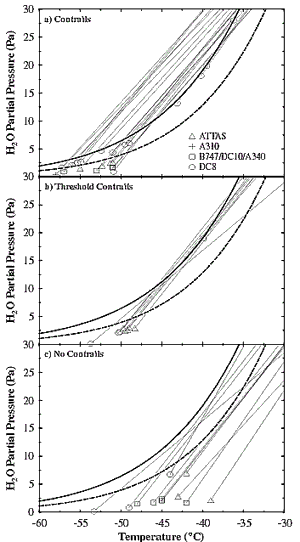
Figure 3-4: Water vapor partial pressure and temperature
measurements and calculations from various contrail studies.
|
Contrails become visible within roughly a wingspan distance behind the aircraft,
implying that the ice particles form and grow large enough to become visible
within the first tenths of a second of plume age. Ice size distributions peak
typically at 0.5 to 1 µm number mean radius (Figure 3-2).
A lower limit concentration of about 104 cm-3 of ice-forming particles in the
plume (at plume ages between 0.1 and 0.3 s) is necessary for a contrail to have
an optical depth above the visibility threshold (Kärcher et al., 1996b). These
values and the corresponding mean radii of 1 µm of contrail ice particles are
in agreement with in situ measurements in young plumes (Petzold et al., 1997).
Initial ice particle number densities increase from 104 to 105 cm-3 and mean
radii decrease from 1 to 0.3 µm when the ambient temperature is lowered by 10
K from a typical threshold value of 222 K (Kärcher et al., 1998a). Although
aerosol and ice particle formation in a contrail are influenced by the fuel
sulfur content (Andronache and Chameides, 1997, 1998), it has only a small (<
0.4 K) impact on the threshold temperature for contrail formation (Busen and
Schumann, 1995; Schumann et al., 1996).
Simulations of contrail formation further suggest that contrails would also
form without soot and sulfur emissions by activation and freezing of background
particles (Jensen et al., 1998b; Kärcher et al., 1998a). However, the resulting
contrails would have fewer and larger particles.
Ice particle size spectra within and at the edge of young contrails systematically
differ from each other (Petzold et al., 1997). Ambient aerosol may play a larger
role in contrail regions that nucleate at the plume edges, where the ratio of
ambient to soot particles is largest and when ambient temperatures are low (212
K) (Jensen et al., 1998b). Ice particles may also nucleate on ambient droplets
in the upwelling limbs of vortices and could contribute to contrail ice mass
(Gierens and Ström, 1998). Metal (and soot) particles have been found as inclusions
in contrail ice particles larger than 2 to 3 µm in radius (Twohy and Gandrud,
1998), but these particles are numerically unimportant compared with other plume
particles.
Contrail ice crystals evaporate quickly when the ambient air is subsaturated
with respect to ice, unless the particles are coated with other species such
as HNO3 (Diehl and Mitra, 1998). Simulations suggest that a few monolayers of
HNO3 may condense onto ice particle surfaces and form NAT particles in stratospheric
contrails (Kärcher, 1996). These particles would be thermodynamically stable
and longer lived and would cause a different chemical perturbation than would
short-lived stratospheric contrails composed of water ice. However, the relevance
of this effect on larger scales has not yet been studied because no parameterization
of NAT particle nucleation in aircraft plumes exists for use in atmospheric
models (Chapter 4).
3.2.4.2. Freezing of Contrail Particles
In a young contrail, activated particles first grow to sizes > 0.1 µm by water
uptake before many of them freeze homogeneously to form water-ice particles
(Kärcher et al., 1995; Brown et al., 1997). The fraction of H2SO4/H2O droplets
that freezes depends on the actual droplet composition, which affects the homogeneous
freezing rate, the time evolution of H2O supersaturation and temperature in
the plume, and possible competition with heterogeneous freezing processes involving
soot (see Figure 3-1).
Pure water droplets freeze homogeneously (without the presence of a foreign
substrate) at a rate that grows in proportion to droplet volume and becomes
very large when the droplet is cooled to the homogeneous freezing limit near
about -45°C (Pruppacher, 1995). Acidic solutions freeze at lower temperatures
than pure water. Freezing is often induced heterogeneously by solid material
immersed inside a droplet (immersion freezing) or in contact with its surface
(contact freezing). Prediction of heterogeneous freezing rates requires detailed
knowledge about the ice-forming properties of droplet inclusions (Pruppacher
and Klett, 1997). If homogeneous and heterogeneous freezing processes are possible,
the most efficient freezing mode takes up available H2O and may prevent the
growth of other particle modes.
|
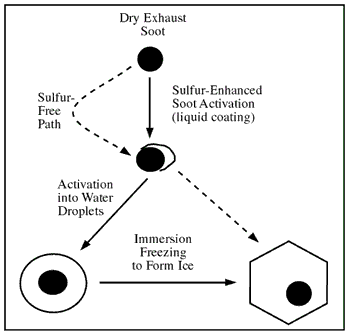
Figure 3-5: Soot activation and heterogeneous freezing in
young aircraft exhaust plumes (Kärcher, 1998a).
|
When the ambient temperature is near the threshold value for contrail formation,
models suggest that volatile aerosol particles take up only a little water and
stay below the critical size (radii > 2 to 5 nm) required for growth and subsequent
freezing (Kärcher et al., 1995). This critical size-hence freezing probability-depends
on maximum supercooling reached in the expanding plume. Particle growth rates-hence
freezing rates-are larger in cooler and more humid ambient air, so volatile
particles may contribute considerably to ice crystal nucleation at temperatures
below the contrail threshold value. This result is supported by observations
of contrails and their microphysical properties for different fuel sulfur levels
(Petzold et al., 1997) and environmental temperatures (Freudenthaler et al.,
1996). Volatile particles grown on charged droplets are more easily activated
than neutral mode droplets (compare in Figure 3-2),
therefore may play an enhanced role in contrail formation (Yu and Turco, 1998b).
Ambient particles also contribute to ice crystal nucleation in the contrail
(Twohy and Gandrud, 1998).
In contrail particle formation, heterogeneous freezing processes involving
soot (see Figures 3-1 and 3-5)
compete with homogeneous freezing of volatile plume particles. Volatile droplets
will be prevented from freezing if rapid freezing of soot-containing particles
occurs. Although this analysis is supported by model simulations and some observations
(Gierens and Schumann, 1996; Kärcher et al., 1996b, 1998a; Schumann et al.,
1996; Brown et al., 1997; Konopka and Vogelsberger, 1997; Schröder et al., 1998a;
Twohy and Gandrud, 1998), the freezing probability of soot is poorly known because
unique evidence that soot is directly involved in ice formation is difficult
to obtain from in situ measurements. On the other hand, fresh soot particles
do not act as efficient ice (deposition) nuclei in the exhaust (Rogers et al.,
1998), consistent with the absence of contrails at temperatures above the liquid
water saturation threshold.
Contrails observed near threshold formation conditions are thought to result
from freezing of water on soot particles (Kärcher et al., 1996b; Schumann et
al., 1996; Brown et al., 1997) (Figure 3-5). This result
is supported by laboratory experiments (DeMott, 1990; Diehl and Mitra, 1998)
that provide evidence that soot may induce ice formation by heterogeneous immersion
freezing at temperatures colder than about 250 K. Water activation of soot may
result from the formation of at least a partial surface coating of H2SO4/H2O
droplets, which likely develops for average to high fuel sulfur levels (Figure
3-5). Hence, more fuel sulfur leads to a greater number of ice particles.
However, observations demonstrate that the number of ice particles (diameter
> 300 nm) in young contrails increases by only about 30% when the fuel content
increases from 6 to 2700 ppm (Petzold et al., 1997), as model simulations of
contrail formation also show (Kärcher et al., 1998a).
Contrails at threshold conditions appear to be formed for very low (2 ppm)
fuel sulfur content in the same manner as for average fuel sulfur content (260
ppm) (Busen and Schumann, 1995), but their properties differ measurably for
larger fuel sulfur content (Schumann et al., 1996). This result suggests that
soot may take up water even at zero fuel sulfur content, though this uptake
may be enhanced in the presence of sulfur emissions (Kärcher et al., 1998a;
see Figure 3-5).
The presence of liquid coatings may alter the chemical reactivity of dry exhaust
soot, which is poorly known (Chapter 2). Soot particles
acting as freezing nuclei have the potential to alter cirrus cloud properties
(see Section 3.4). Present observations do not rule out
the possibility that aircraft soot particles can act as freezing nuclei in cirrus
formation, perhaps even without a H2SO4/H2O coating. Information is lacking
on how the chemical reactivity and freezing properties of soot might change
in aging plumes from interactions with background gases and particles or as
a result of aerosol processing in contrails.
Table 3-2: Emission indices and estimated global emission
rates of exhaust products of the present (1992) aircraft fleet using representative
emission indices. Emission sources other than aircraft and estimated magnitudes
of these emissions are listed in the last two columns. Values in parentheses
indicate estimated range (adapted from Fabian and Kärcher, 1997; Schumann,
1994).
|
Fuel and
Emissions |
Emission Index
(g pollutant/kg fuel) |
Emission Rate
(1992 fleet)
(Tg yr-1) |
Comparable Emission
(Tg yr-1) |
Comparable Emission Source |
| |
|
|
|
|
| Fuel |
- |
140 (139-170)a |
3140 |
Total consumption of petrol |
| |
|
|
|
|
| H2O |
1260 |
176 |
45 |
CH4 oxidation in the stratosphere |
| |
|
|
525000 |
Evaporation from Earth's surface |
| |
|
|
|
|
| NOx (as NO2) |
14 (12-16)a |
2 |
2.9 ± 1.4 |
Flux from the stratosphere |
| |
|
|
90 ± 35 |
All anthropogenic sources |
| |
|
|
|
|
| Soot burning |
0.04 (0.01-0.1)b |
0.006 |
12c |
Fossil fuel combustion and biomass |
| |
|
|
|
|
| Sulfur |
0.4 (0.3-0.5) |
0.06 |
65d |
Total from fossil fuel combustion |
| |
|
|
10-50 |
Natural source, mostly as DMSe |
| |
|
|
2.7f |
Non-eruptive volcanoes |
| |
|
|
4.0g |
Eruptive volcanoes |
| |
|
|
|
|
| CxHy surface |
0.6 (0.2-3.0) |
0.1 |
90 |
Anthropogenic emissions at Earth's |
|
a) From Chapter 9.
b) Döpelheuer, 1997.
c) Liousse et al., 1996.
d) Benkovitz et al., 1996.
e) Watson et al., 1992.
f) Spiro et al., 1992.
g) Chin et al., 1996. |
|
|