|
3.2. Aerosol Emission and Formation in Aircraft Plumes
Aircraft jet engines directly emit aerosol particles and condensable gases
such as water vapor (H2O), sulfuric acid (H2SO4), and organic compounds, which
lead to the formation of new, liquid (volatile) particles in the early plume
by gas-to-particle conversion (nucleation) processes. Other gas-phase species
and charged molecular clusters (chemi-ions, or CIs) are also generated at emission,
including nitric acid (HNO3) and nitrous acid (HNO2). Emission and formation
of H2SO4 depend on fuel sulfur content, or sulfur emission index [EI(S)], and
the conversion fraction of fuel sulfur to H2SO4. Formation of HNO3 and HNO2
depends on reactions of nitrogen oxides (NOx = NO + NO2) with hydroxyl radicals
(OH). Particle formation depends on mixing of exhaust gases with ambient air,
plume cooling rate, plume chemistry, and ambient aerosol properties. Soot particles
formed during fuel combustion and emitted metallic particles constitute the
solid (nonvolatile) particle fraction present in exhaust plumes. Under certain
thermodynamic conditions, emitted water vapor condenses and freezes to form
water-ice particles, thereby producing a condensation trail (contrail). These
line clouds evaporate rapidly if the ambient humidity is low but may change
the size and chemical composition of the remaining liquid aerosol particles.
If the humidity is above ice saturation, contrails persist and grow through
further deposition of ambient water.
An invisible aerosol trail is always left behind cruising aircraft. Aerosol
and contrail formation processes in an aging plume determine the number, surface
area, and mass of particles that are formed per mass of fuel consumed. Exhaust
particle properties change in the presence of a contrail. Exhaust particle morphology
and surface properties and aircraft-induced perturbations of background aerosol
surface areas (Section 3.3) are of central importance
for ozone changes caused by heterogeneous chemical reactions (Chapters 2
and 4). Particle number and freezing probability are
key for the formation of ice (cirrus) clouds after passage of an aircraft in
a region where otherwise no clouds would form (Section 3.4).
Finally, aviation-produced aerosol can directly or indirectly influence the
radiation budget of the atmosphere (Section 3.6 and Chapter
6). For recent reviews see Schumann (1996a), Fabian and Kärcher (1997),
Friedl (1997), and Brasseur et al. (1998).
The following subsections provide a description of volatile aerosol precursors
and the formation of volatile aerosol particles, a characterization of emitted
soot and metal particles, a review of contrail and ice formation, and a discussion
of the mutual interactions between these particle types. Comments on reducing
the impact of aerosols are given in Section 3.7.4.
3.2.1. Volatile Aerosol Precursors
3.2.1.1. Water Vapor
Water vapor is present in aircraft exhaust in known amounts because the emission
index is specified by the stoichiometry of near-complete fuel combustion (Chapter
7). Water vapor concentrations of a few percent at the engine exhaust nozzle
far exceed the concentrations of other aerosol precursor gases. Ambient water
vapor also participates in aerosol processes, with concentrations that vary
widely depending on flight altitude and meteorological processes. Because of
its abundance and thermodynamic properties, water vapor participates in nearly
all aerosol formation and nucleation processes (e.g., Pruppacher and Klett,
1997).
3.2.1.2. Sulfur Species
Aviation fuels (kerosene) contain sulfur in trace amounts. In the current world
market, the sulfur content-hence the EI(S)-of aviation fuels is near 0.4 g S/kg
fuel or 400 parts per million by mass (ppm; 1 ppm = 0.0001%), with an upper
limit specification of 3 g S/kg (Chapter 7). Of importance
for the formation of plume aerosol is the partitioning of sulfur at the engine
exhaust nozzle into sulfur dioxide (SO2) and fully oxidized sulfur S(VI) compounds,
sulfur trioxide and sulfuric acid (S(VI) = SO3 + H2SO4). Most fuel sulfur is
expected to be emitted as SO2 based on combustion kinetics and some observations
(Miake-Lye et al., 1993, 1998; Arnold et al., 1994; Schumann et al., 1998).
However, a fraction of the SO2 can be converted into S(VI) by gas phase chemical
reactions with OH, oxygen atoms (O), and H2O inside the engine. The fractional
conversion depends on details of combustion conditions, turbine flow properties,
blade cooling effects (Chapter 7), and mixing (Chapter
2). Further oxidation can occur in the plume, where the rate-limiting step
is thought to be oxidation of SO2 by OH to form SO3 (Stockwell and Calvert,
1983) or liquid-phase reactions of SO2 with H2O2, O3, metals (Jacob and Hofmann,
1983), or HNO3 (Fairbrother et al., 1997). Once SO3 is formed, the gas-phase
reaction with emitted H2O to form H2SO4 is fast (< 0.1 s) under plume conditions
(Reiner and Arnold, 1993; Kolb et al., 1994; Lovejoy et al., 1996). Gaseous
H2SO4 and HSO4-(H2SO4)n (mostly with n = 1,2) ion clusters have been observed
in jet exhaust (Frenzel and Arnold, 1994; Arnold et al., 1998a,b).
|
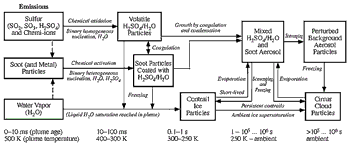
Figure 3-1: Aerosol and contrail formation processes in an aircraft plume and
wake as a function of plume age and temperature.
|
The chemical lifetime of exhaust OH in the early jet regime is determined by
reactions with emitted NOx and by OH self-reactions, the latter leading to the
formation of hydrogen peroxide (H2O2) (Kärcher et al., 1996a; Hanisco et al.,
1997). Measurements indicate OH exit concentrations below 1 ppmv (Tremmel et
al., 1998). For an OH concentration of 0.5 to 1 ppmv at the engine's nozzle
exit plane and without SO3 emissions, the OH-induced pathway alone yields about
0.3 to 1% S-to-H2SO4 conversion in the plume (Miake-Lye et al., 1993; Danilin
et al., 1994; Kärcher et al., 1996a). Model calculations indicate overall S(VI)
conversion fractions in the range of 2 to 10% for various supersonic and subsonic
jet engines (Brown et al., 1996a; Lukachko et al., 1998; Chapter
7), consistent with some earlier SO3 measurements behind gas turbines (e.g.,
CIAP, 1975; Hunter, 1982).
3.2.1.3. Chemi-ions
A large number of chemi-ions (CIs) are expected to be present in aircraft exhaust
because ion production via high-temperature chemical reactions is known to occur
in the combustion of carbon-containing (not necessarily sulfur-containing) fuels
(e.g., Burtscher, 1992). In the jet regime, some recent models indicate that
CIs effectively promote formation and growth of electrically charged droplets
containing H2SO4 and H2O (Yu and Turco, 1997). In addition, CIs may contribute
to the activation of exhaust soot. Positive ions include H3O+ and organic molecules
like CHO+, C3H3+, and larger molecules (Calcote, 1983), whereas the free electrons
rapidly attach to other molecules to form negative ions with sulfate and nitrate
cores. Measurements of positive CIs in exhaust plumes are not available, and
only very few in situ measurements of negative CIs are available to date.
Arnold et al. (1998a) measured a total negative CI concentration of 3 x 107cm-3 (about 3 x 1015/kg fuel) at plume ages of around 10 ms in the exhaust of
a jet engine on the ground, consistent with approximately 109 cm-3 at a plume
age of 1 ms (Yu et al., 1998).
That concentration represents a lower bound from diffusion losses of these
particles within sampling devices prior to detection and the limited detection
range of the employed mass spectrometer. The fact that these measurements yielded
only a fraction of CIs in the plume has been partially confirmed by in-flight
measurements (Arnold et al., 1998b) showing smaller total CI count rates for
high-sulfur fuel compared with low-sulfur fuel. Therefore, current CI data are
consistent with a CI emission index of about 2-4 x 1017/kg, corresponding to
a concentration of about 2 x 109 cm-3 at the engine exit. This value has been
estimated numerically based on coupled ion-ion recombination kinetics and plume
mixing (Yu and Turco, 1997; Kärcher et al., 1998b; Yu et al.,1998). Although
not directly comparable, CIs have been observed in hydrocarbon flames at concentrations
of about 108 to 1011 cm-3 (Keil et al., 1984), supporting the estimated concentration
of negative CIs.
3.2.1.4 Nitrogen Species
The primary nitrogen emission from aircraft is in the form of NOx (Chapter
2). In reactions of NOx with OH in the plume, gaseous HNO2 and HNO3 are
formed. Despite larger reaction rates, less HNO3 is formed than HNO2 because
the ratio of NO2 to NO at the engine exit is small (< 0.2) (e.g., Schulte et
al., 1997) (see Chapter 7). HNO3 can also form in the
plume even in the absence of NO2 emissions (Kärcher et al., 1996a). In situ
measurements in young plumes revealed both HNO2 and HNO3 concentrations above
background levels (Arnold et al., 1992, 1994; Tremmel et al., 1998). HNO3 can
be more abundant in plumes than H2SO4, especially for low EI(S) values. These
acids (especially HNO3) are important because they can be taken up by water-soluble
exhaust particles and form stable condensed phases such as nitric acid trihydrate
(NAT = HNO33H2O) and liquid ternary (H2O/H2SO4/HNO3) solutions under cold and
humid plume conditions (Arnold et al., 1992; Kärcher, 1996).
3.2.1.5. Hydrocarbons
Aircraft engines emit non-methane hydrocarbons (NMHCs) as a result of incomplete
fuel combustion. These species include alkenes (mostly ethene), aldehydes (mostly
formaldehyde), alkines (mostly ethine), and a few aromates. A few (8 to 10)
species were found to account for up to 80% of NMHC emissions (Spicer et al.,
1992, 1994). High levels of carbonyl compound emissions (on the order of 0.2
ppmv) also have been observed in a combustor (Wahl et al., 1997). Some in-flight
data indicate that NMHCs with up to 8 carbon atoms have EIs in the range 0.05
to 0.2 g C/kg fuel and represent approximately 70% of total NMHC emissions (Slemr
et al., 1998). However, the current database on NMHC emissions and on partitioning
between individual compounds is small and perhaps not representative for all
engine types. Some emitted NMHCs might act as aerosol-forming agents in nascent
plumes and may be adsorbed or dissolved in plume particles, thereby possibly
contributing to the total amount of volatile aerosol found in plumes (Kärcher
et al., 1998b). In addition, the presence of trace NMHCs amounts may facilitate
nucleation (e.g., Katz et al., 1977) and alter the hygroscopic behavior and
growth rates of particles (Saxena et al., 1995; Cruz and Pandis, 1997). Engines
also may emit volatile particles containing engine oils or other lubricants,
but this effect has not been quantified.
Aircraft also occasionally introduce hydrocarbons by jettisoning fuel at low
altitudes in the troposphere. Most of the fuel evaporates while it falls to
the ground (Quackenbush et al., 1994), which leads to a small increase of hydrocarbons
in this region. Because of the small amounts of fuel released in this way, no
essential impacts on atmospheric aerosols are expected.
3.2.2. Volatile Particles
3.2.2.1. Basic Processes
Volatile particles form in the exhaust plume of an aircraft as a result of
nucleation processes associated with the emission of aerosol precursors (Hofmann
and Rosen, 1978). Typical aerosol parameters in a young plume are included in
Table 3-1 for reference. The newly formed particles
grow by condensation (uptake of gaseous species) and coagulation (particles
collide and attach) in the expanding plume. Coagulation processes involving
charged particles originating from CI emissions are more effective because charge
forces enhance collision rates. These processes are schematically presented
in Figure 3-1. Key processes at young plume ages
are determined mainly from the results of simulation models because of the lack
of suitable plume measurements.
Previous models (Miake-Lye et al., 1994; Kärcher et al., 1995; Zhao and Turco,
1995; Brown et al., 1996b; Taleb et al., 1997; Gleitsmann and Zellner, 1998a,b)
show that particles form when the condensing species, primarily H2SO4/H2O, reach
concentrations critical for binary homogeneous nucleation in the expanding and
cooling exhaust gas. Because concentrations fall below nucleation thresholds
as the plume further dilutes, the amount of H2SO4 in the early stages of the
plume (< 1 s) controls the formation of new volatile particles. More recent
models emphasize the role of CI emissions in volatile particle formation and
growth (Yu and Turco, 1997, 1998a,b; Kärcher, 1998a; Kärcher et al., 1998a).
Figure 3-2 shows the size distributions of exhaust
particles at a plume age of 1 s, as inferred from models and a few measurements.
In the radius range below 10 nm, volatile particles containing H2SO4 and H2O
dominate the overall distribution. Model analyses of near-field particle measurements
strongly suggest that the volatile particle size distribution exhibits a bimodal
structure (Yu and Turco, 1997), with smaller particles formed by the aggregation
of homogeneously nucleated clusters of hydrated H2SO4 molecules (neutral mode)
and larger particles formed by rapid scavenging of charged molecular clusters
by CIs (ion-mode). Only particles from the ion mode are expected to grow beyond
the smallest detectable sizes (radius ~2 to 3 nm) of particle counters. Soot
and ice contrail particles are significantly larger than the volatiles. An approximate
stratospheric size distribution is shown for comparison. In contrast to soot
and ice particles (see below), volatile particle spectra are mainly derived
from numerical simulation models because of the lack of size-resolved, in situ
particle measurements in the nanometer size range. However, the use (in field
measurements) of multiple particle counters with different lower size-detection
limits allows derivation of the mean sizes of observable particles as a function
of fuel sulfur content and plume age (Kärcher et al., 1998b; Yu et al., 1998),
thereby providing strong criteria to test the validity of model results.
Table 3-1: Summary of number mean radius, number density,
and surface area density for sulfate and soot particles in aircraft plumes
and in the background atmosphere, and for ice particles in contrails and
cirrus. Flight levels of subsonic (supersonic) aircraft are in the 10-12
km (16-20 km) range. Also included are estimates of zonal mean perturbations
to sulfate and soot properties caused by the 1992 aircraft fleet.
|
| Density |
Radius
(mm) |
Number Density
(cm-3) |
Surface Area Density
(mm2 cm-3) |
| |
|
|
|
| Sulfate |
|
|
|
| Plume (1 s)a |
0.002 |
(1-2) x 107 |
500-1000 |
| Background (10-12 km)b |
0.01-0.1 |
50-1000 |
1-6 (10-40) |
| Background (20 km, non-volcanic)c |
0.07 |
5-10 |
0.5-1 |
| Background (20 km, volcanic)d |
0.2-0.5 |
10-100 |
10-40 |
| |
|
|
|
| Soot |
|
|
|
| Plume (1 s)e |
0.01-0.03 |
5 x 104- 5 x 104 |
50-5000 |
| Background (10-20 km)f |
0.05-0.1 |
0. 01-0.1 |
3 x 10-5- 3 x 10-2 |
| |
|
|
|
| Ice |
|
|
|
| Young Contrail (0.1-0.5 s)g |
0.3-1 |
104-105 |
104-105 |
| Persistent Contrail (10 min to 1 h)h |
1-15 |
10-500 |
103-104 |
| Young Cirrusi |
5-10 |
1 |
102-104 |
| |
|
|
|
| 1992 Aircraft Perturbationj |
|
|
|
| Sulfate Aerosol (10-12 km, 50-60°N) |
0.01 |
90-900 |
0.1-1.1 |
| Soot (10-12 km, 50-60°N) |
0.02 |
3-30 |
0.02-0.2 |
|
|
a) Detectable only by ultrafine particle counters (particles
smaller than 2-3 nm radius are not detected). Calculations by Yu and Turco
(1997) for average FSC consistent with observed data.
b) Properties highly variable; size distributions often bimodal.
Ranges include small (> 10 nm) particles. Large particle mode (~100 nm)
often similar to stratospheric aerosol particles (Hofmann, 1993; Yue et
al., 1994; Schröder and Ström, 1997; Solomon et al., 1997; Hofmann et al.,
1998). High range of values inferred from satellite extinction data and
represents mixtures of aerosols and subvisible clouds.
c), d) Yue et al., 1994; Kent et al., 1995; Borrmann et al., 1997;
Thomason et al., 1997b.
e) Hagen et al., 1992; Rickey, 1995; Petzold et al., 1999.
f) Only largest soot particles with longest atmospheric lifetimes
are measured by wire impactors (Sheridan et al., 1994; Blake and Kato, 1995;
Pueschel et al., 1997). Uncertainties in total surface area introduced by
fractal geometry of particles.
g) Kärcher et al., 1996b, 1998a; Petzold et al., 1997.
h) Values representative of contrail core for low ice-supersaturation
(Heymsfield et al., 1998a; Schröder et al., 1998b) (see also Sections
3.4.4 and 3.6.3). Far larger particles are
observed for large ice-supersaturation (Knollenberg, 1972; Gayet et al.,
1996).
i) Ström et al., 1997; Schröder et al., 1998b. Larger values are
observed in warm cirrus clouds (Heymsfield, 1993; see also Sections
3.4.4 and 3.6.3).
j) Results of fuel tracer simulations discussed in Section 3.3.4.
Values shown represent upper bounds to zonal mean perturbations caused by
emissions of the 1992 aircraft fleet. Results are representative of flight
levels at northern mid-latitudes and are calculated using the range of values
of computed tracer concentrations from all models and assuming a fuel sulfur
content of 0.4 g/kg fuel, a 5% conversion of sulfur to sulfate aerosol,
an EI(soot) of 0.04 g/kg fuel, and a mean particle size of 10(20) nm for
sulfate (soot) particles. |
|
Once formed, the new volatile particles interact with nonvolatile and contrail
ice particles through the processes of coagulation, freezing, condensation,
and evaporation (Figure 3-1). Calculations
show that the new liquid particles grow and shrink as a function of relative
humidity, whereas H2SO4
molecules that enter the droplets stay in the liquid phase because of their
very low saturation vapor pressure (Mirabel and Katz, 1974). They also suggest
that volatile particles may take up HNO3 and
H2O in the near field (Kärcher, 1996) to form
particles with compositions similar to those found in cold regions of the stratosphere.
These particles may persist in cold (< 200 K), HNO3-rich
stratospheric air but will be short-lived (< 1 min) otherwise. As the plume
continues to dilute with ambient air, abundant newly formed volatile particles
remain at nanometer sizes and therefore add substantially to the overall aerosol
surface area and abundance (Danilin et al., 1997). Their efficiency for heterogeneous
chemistry and cloud formation, however, is size- and composition-dependent (Kärcher,
1997). They may be too small to act as efficient cloud- or ice-forming nuclei
in the background atmosphere unless the air mass containing the aerosol is lifted
or cooled or the relative humidity increases. Although studies exist on heterogeneous
plume processing along selected trajectories (Danilin et al., 1994), systematic
investigations of heterogeneous chemistry coupled to plume aerosol dynamics
remain to be performed (Chapter 2).
|
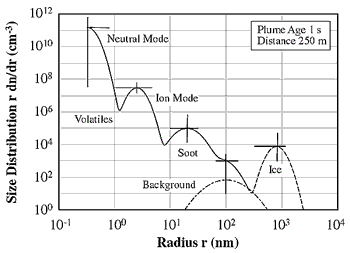
Figure 3-2: Size distribution of various aerosol types present in
young jet aircraft exhaust plumes (adapted from Kärcher, 1998a).
|
The evolution of volatile particles is significantly altered if a contrail
forms. In contrails, volatile particles have to grow to sizes greater than about
100 nm via uptake of ambient H2O before most
of them freeze (Section 3.2.4.2). As ice particles
grow in size by deposition of H2O, they may also
scavenge other volatile and soot particles (Anderson et al., 1998a,b; Schröder
et al., 1998a). Thus, contrails are expected to contain fewer small particles
than non-contrail plumes because of enhanced scavenging losses. After evaporation
of contrail ice crystals, the residual volatile and soot cores remain as particles
in the atmosphere (Figure 3-1). This contrail
processing is expected to modify the particle size distribution and composition
and may lead to efficient cloud condensation nuclei production (Yu and Turco,
1998b).
3.2.2.2. Observations and Modeling of Volatile Particles
and Sulfur Conversion
Volatile particle abundances observed in situ in the plumes (mostly young,
< 100 s) of subsonic and supersonic aircraft are summarized in Figure
3-3a. The data have been compiled from various field studies (Fahey et al.,
1995a,b; Schumann et al., 1997; Anderson et al., 1998a,b; Schröder et al., 1998a).
The results show EIs for ultrafine volatile aerosol particles (nominal radii
> 2 to 3 nm) in the range of 1015 to 1016/kg fuel for low to average fuel sulfur
content values and exceeding 1017/kg fuel for high-sulfur fuel. Besides the
obvious dependence on fuel sulfur content, the spread in EI values may be explained
by differences in the emission characteristics of the engines, variations in
the lower size detection limits of the particle counters, and differences in
plume ages at the time of the observations. The increase in ultrafine particle
abundance with increasing fuel sulfur content for the Advanced Technology Testing
Aircraft System (ATTAS), T-38, and B757 aircraft strongly suggests an important
role for fuel sulfur in the growth of volatiles from molecular clusters to detectable
particles.
Only a few observations have been analyzed using detailed microphysical simulation
models (Brown et al., 1996a; Danilin et al., 1997; Kärcher and Fahey, 1997;
Yu and Turco, 1997, 1998a; Andronache and Chameides, 1998; Kärcher et al., 1998a,b).
Simulations show better agreement between calculated and observed particle concentrations
when ion effects are taken into account. More important, the description of
plume microphysics using binary homogeneous nucleation failed to explain a field
measurement (Yu et al., 1998). In two cases, condensation nucleus observations
in the exhaust of the ATTAS and the Concorde (Figure 3-3a)
have been explained in detail with a model that includes CI emissions on the
order of 1017/kg fuel. The observable (ion mode) particles have mean radii of
about 2 to 4 nm in the young plume, for EI(S) ranging from average to high values.
For decreasing levels of available H2SO4, the ion mode particles decrease in
size. The number of detected particles falls below 1017/kg fuel when the mean
radius of their size distribution becomes smaller than the detection limit of
the particle counters.
|
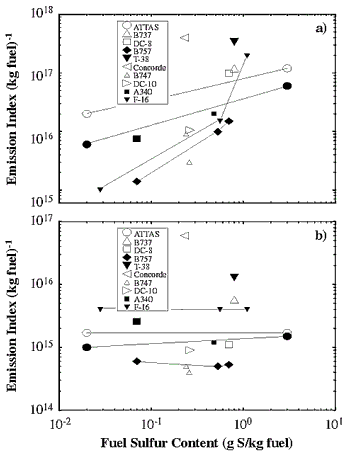
Figure 3-3: (a) Emission indices of detectable volatile particles in
number per kg fuel measured in situ in plumes of various subsonic aircraft and the
supersonic Concorde. (b) Same as (a), but for the emission indices of soot particles.
|
The extent of conversion of fuel sulfur to S(VI) necessary to explain the observed
mass of volatile aerosol in young plumes seems to be variable. Direct measurements
of H2SO4 have provided a lower bound of ~0.4% (for high-sulfur fuel, 2.7 g/kg
fuel) and an upper bound of ~2.5% (for low-sulfur fuel, 0.02 g/kg fuel) for
the conversion fraction in one case (Curtius et al., 1998), consistent with
calculated SO3 emission levels (Brown et al., 1996a,c). For the low fuel-sulfur
case, it has been demonstrated that the observed volatile particles cannot be
mainly composed of H2SO4 (Kärcher et al., 1998b). In other cases, conversion
fractions have been indirectly inferred from mass balance arguments involving
observed or inferred particle size and number distributions, available sulfur
as measured in fuel samples, and assumptions of aerosol composition. The dependence
of the conversion fraction on EI(S) differs in the few studies performed to
date (Fahey et al., 1995a; Schumann et al., 1996; Hagen et al., 1998; Miake-Lye
et al., 1998; Pueschel et al., 1998). The values deduced from these measurements
range from the minimum value of 0.4% to more than 20%. Some of the indirect
analyses may be affected by uncertainties (possibly about 20%) regarding the
sulfur content in the fuels. Other experimental uncertainties are associated
with these determinations, and the range of aircraft engines and operating parameters
adds to the observed variability.
Generalization of these results cannot be done with confidence because of limited
empirical knowledge of the S(VI) conversion fraction and emission levels of
CIs representative of the exhaust as it enters the atmosphere. Part of the observed
volatile aerosol may be composed of HNO2 (Zhang
et al., 1996; Kärcher, 1997) or NMHCs (Kärcher et al., 1998b), or it may result
from unrecognized sulfur oxidation reactions (Danilin et al., 1997; Miake-Lye
et al., 1998). Uptake of gaseous SO2 and subsequent
heterogeneous oxidation to sulfate in the new volatile particles is likely small
(Kärcher, 1997), but the possibility of other condensational or aqueous-phase
growth mechanisms has not yet been fully explored. On the other hand, CI emissions
of about 1017/kg fuel are consistent with observations (see Section
3.2.1.3), although they need to be confirmed by further measurements at
the engine exit. The best estimate for the S-to-H2SO4
conversion fraction in young plumes is 5%, with an estimated variability, or
uncertainty range, of 1 to 20%. Combustion models predict a range of sulfur
conversion up to about 10% and a potential for slightly higher values because
of turbine blade cooling effects (Sections 3.2.1.2
and 7.6). Low conversion fractions of 2% are sufficient
to explain observed volatile particle concentrations in the young plume behind
the ATTAS when the effects of CIs are taken into account in simulation models
of plume chemistry and microphysics (Kärcher et al., 1998a,b; Yu and Turco,
1998a,b; Yu et al., 1998). In contrast, the Concorde observations can be explained
by assuming that about 20% of the fuel sulfur is converted to SO3
before leaving the engine exit in such simulations (Yu and Turco, 1997).
In situ measurements detailing particle volatility and size distributions
such as those included in Figure 3-3 have involved
relatively young plumes. Further observations in aging plumes (> 1 h) as they
dilute with the background atmosphere are currently lacking. Without detailed
observations of the microphysical evolution and chemical composition of volatile
exhaust particles from the engine exhaust plume to the global scale, important
uncertainties remain in assessing the potential global impact of exhaust products
on chemistry and cloudiness.
|